About Us Approval Notification Guidelines Activities FAQ
Contained Use
Application
1. Apply to exportation of living modified organisms.
2. Contained use involving living modified organisms.
3. Importation of living modified organisms for purposes of undertaking a contained use activity.
Submittion of Notification
The notification shall be submitted to the Director General through the Institutional Biosafety Committee in the prescribed form and accompanied by the following documents:
1. An emergency response plan.
2. Specific measures for the contained use activity (regulation + IBC).
3. Such other information as may be specified by the Board.
4. Compliant with requirements of importing country (export).
Acknowlegment
Exemption
The First Schedule of the Biosafety (Approval and Notification) Regulations 2010 allows exemptionsfor some types of techniques and contained use activities in relation to LMO posing a very low risk (i.e.contained research activities involving very well understood organisms and processes for creating and studying LMO):
Notification Process
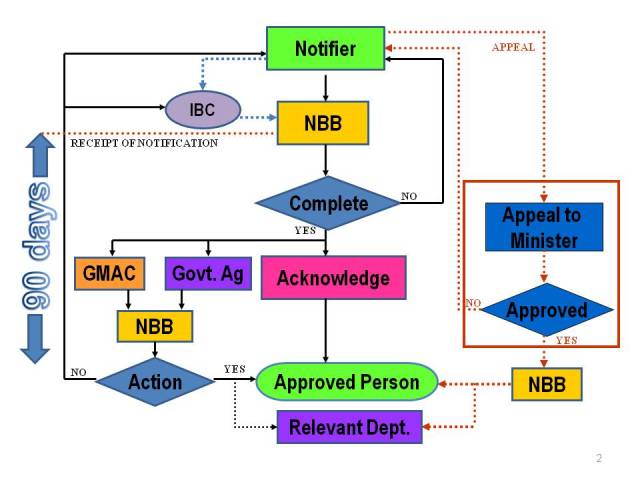
Contained use activities which are exempted from the notification
Biosafety (Approval and Notification) Regulations 2010 (page 20-27)
Last Update: 11/03/2022